
Frequently asked questions
General FAQ
- How can I place an order or receive a quotation?
We work with distributors worldwide to facilitate the logistic process. Please contact the authorized distributor in your area for prices, lead times, and placing your order. Go to find distributor and you will find your local distributor. Orders from the US can be placed in our webshop or send them to orders-us@hycultbiotech.com.
- Can I get a discount?
We offer volume discounts starting from 10 units of the same catalog item. We also offer antibodies in larger bulk sizes starting from 1 mg. In case you would like to receive pricing for a higher volume please contact us or your local distributor
- Is the information in the datasheet and manual up-to-date and correct?
We strive to always provide you with the most up-to-date and correct information available. Please note that the documentation that comes with a product is always leading compared to the product information offered on the website. In case there are any questions on the accuracy or correctness of the information provided in the product information please contact our support team.
- Is the product tested for cross-reactivity?
If relevant and if possible we try to test for cross-reactivity. In case this has been tested we try to mention it in the product information. If you would like to check if a cross-reactivity is tested, please contact us. If you want to determine homology of two proteins, we recommend using the Basic Local Alignment Search Tool: BLAST. A higher homology increases the chances of cross-reactivity. However, we can never guarantee cross-reactivity of a product on a species that has not been validated by us.
- What is the shelf life of Hycult Biotech products?
We guarantee at least 6 months shelf life for ELISA kits and at least one year for antibodies, proteins and bioreagents. Please check the product label for the exact expiry date and recommended storage conditions.
- How can I receive a safety data sheet (SDS)?
There is always an SDS available for ELISA kits on our website. Visit the product page of the ELISA kit in question and download the SDS under the “Downloads” section. If an SDS is not available for other products in our range, please send an e-mail to support@hycultbiotech.com and we will make sure to provide you with the requested documentation as soon as possible.
- Does the product contain any preservatives or carriers?
Antibodies and kit components may contain preservatives to ensure long-term stability and preservation. For most products this is BSA and/or sodium azide. Specific information on preservatives and formulations can always be found on the respective CoA-TDS.
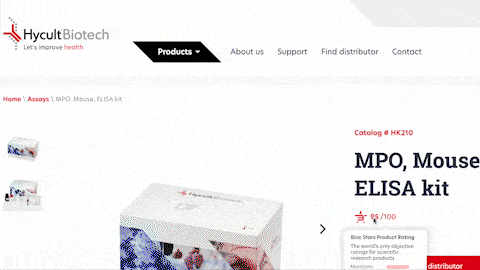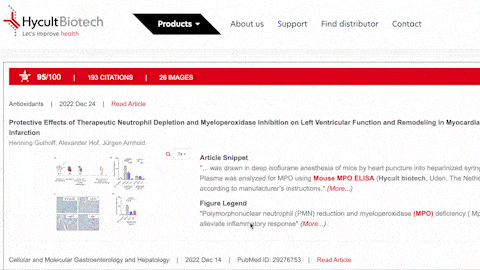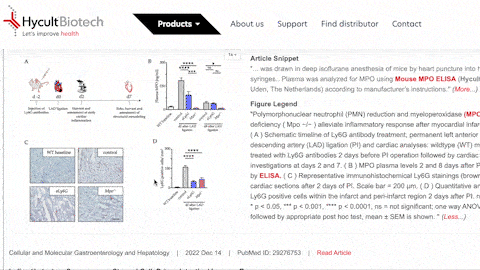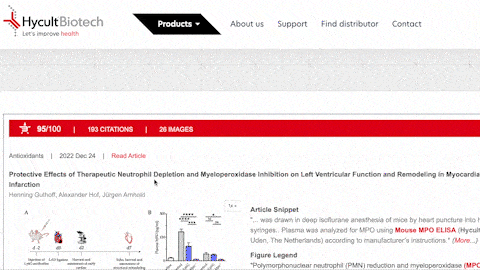
- What is the BIOZ badge explaining?
The badge explains some interresting features since it includes not only the rating and snippets from the article, but also includes images and figure legends, which the users can zoom into. This information is updated daily.
ELISA FAQ
- What curve fitting method is recommended?
Plotting a standard curve can be done using the linear-logarithmic method. We recommend using the Four Parameter Logistic (4PL) regression, which is suitable for creating a standard curve. The curve is constructed by plotting the mean absorbance for each standard on the vertical (Y) axis versis the corresponding concentration on the horizontal (X) axis.
For calculation of the results the straight part of the lin-log curve should be used as this part is the most reliable part of the curve. When the curve is not symmetrical a better fit will be achieved using a 5PL regression. An online resource for curve fitting can be found here Please verify if the software that comes with the ELISA plate reader is capable of performing linear regressions.
- Can I use kit components from two different batches and combine them in one test?
We do not recommend to mix components of two different lot numbers. Every batch is produced individually and components are optimized. Therefore, we cannot guarantee the results if components are mixed from different lots.
Please notice we test each batch for e.g. inter and intra-variation by two different operators with a lot-specific set of components and with samples with a known concentration. All of this is carried out to ensure that the variation between the different batches in minimized.
- Are additional kit components available?
When we produce a new batch there may always be excess material avaible. However, these materials are usually limited. When a particular kit component from a particular lot is required, please contact our technical support to see if we are able to assist.
- What sample type is recommended?
The sample types which are tested and supported by Hycult Biotech are mentioned in the manual. If you wish to test another sample type, please to not hesitate to contact our support team for more information.
- The manual does not mention the sample type that I want to use for testing. Can I use them despite this fact?
If you wish to test a new sample type we recommend to run a recovery experiment. In this way, the influence of components in the sample on the detection of the protein can be determined. Add a known amount of standard into both diluted sample and dilution buffer. Run the samples in duplicate in the assay and calculate the recovery with regards to the normal sample.
The test is valid when the recovery percentage is within the range of 80-120%. If the recovery percentage falls outside this range the samples should be further diluted for a reliable detection of the protein.
- Why are the dilutions mentioned in the manual a minimum dilution?
The dilutions mentioned in the manual have been determined as a minimum dilution to prevent any matrix effect. Depending on the sample type, treatment of the sample or patient status a higher dilution might be required.
- Can a plate washer or an automated workstation be used?
Use of a plate washer or automation may result in higher background and a decrease in sensitivity. We always advise to validate the plate washing method or automation by comparing it with the manual procedure.
- What type of material do you recommend when using tubes, etc.?
We strongly recommend the use of polypropylene (PP) tubes. Polypropylene is a commonly used plastic in laboratory equipment as it has demonstrated to be durable and to have a minimal impact on biological activity. Use of other plastics is not recommended given that this may have an impact on the results.
Pathway assays FAQ
- Can I apply calculation method of existing pathway ELISAs?
Yes, multiple quantification methodology can be applied.
For accurate quantification of complement activity it is advised to apply regression analysis on logistically transformed optical density (OD) values to determine the complement activity as percentage of the activity of the positive reference. This method can be found in following reference: Palarasah Y et al; Novel assays to assess the functional capacity of the classical, the alternative and the lectin pathways of the complement system. Clinical & Experimental Immunology 2011; 164: 388 (http://dx.doi.org/10.1111/j.1365-2249.2011.04322.x)
4PL is possible. Please note, the method is prone to errors and has lower accuracy. Slight differences in dilution factor can lead to large OD differences. Especially when just one or two dilutions are tested. For the standard curve, the obtained OD value should be plotted against the log activity of the reference standard (S1= 100%, S2=50%, S3=25%, S4=12.5%, S5=6.25%).
Semi-quantitative with your own reference sample, the complement activity can be calculated as follows:(OD sample – OD negative control) / (OD own reference sample – OD negative control) x 100%.
- Not all OD values of the 4 suggested sample dilutions are within the OD range of reference standard. Can the result be trusted?
It is advised to base the result on a minimum of 3 dilutions.
If only 1 or 2 dilutions fit within the reference standard, we advise to retest the samples at adjusted dilutions.
- The sample shows an activity > 100%. How should this be interpreted?
It is well-known that complement activity levels in a healthy population is variable between individuals. Our reference sample is a pool of donors which are collected and processed with a dedicated protocol to ensure a fully complement preserved activity status. Therefore it is expected that our reference sample has a different activity status compared to a regular sample in the field. In other words this could lead to a different unit definition. As result the unit definition can differ from other pathway activation assays.
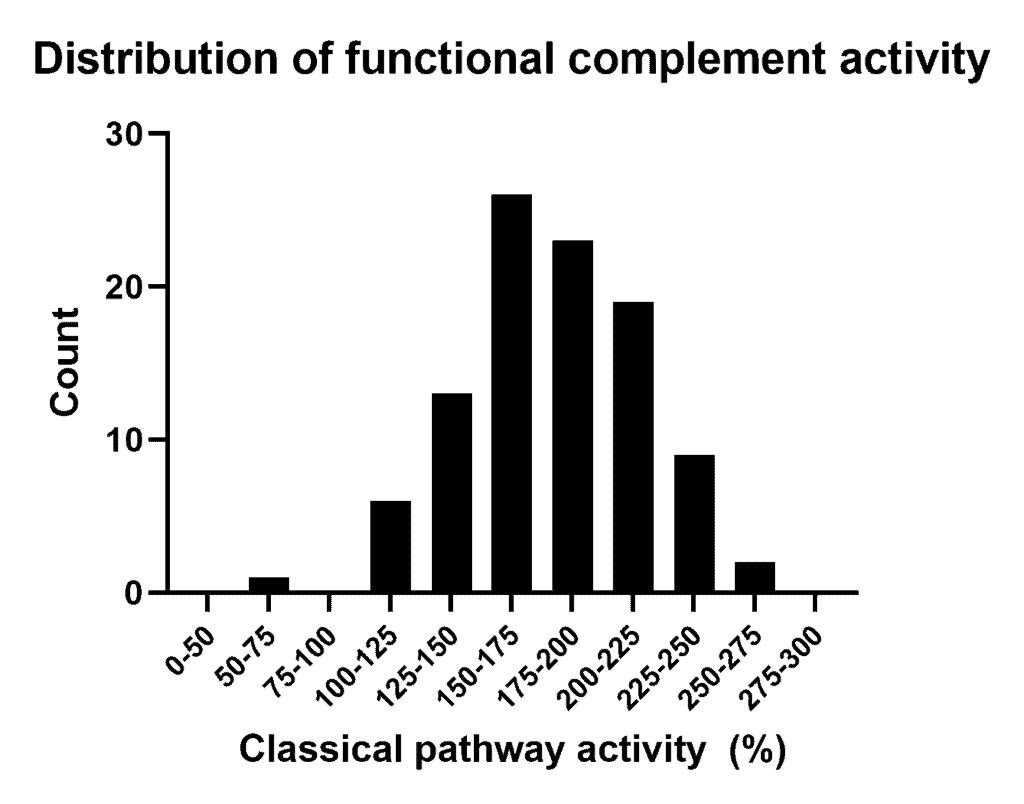
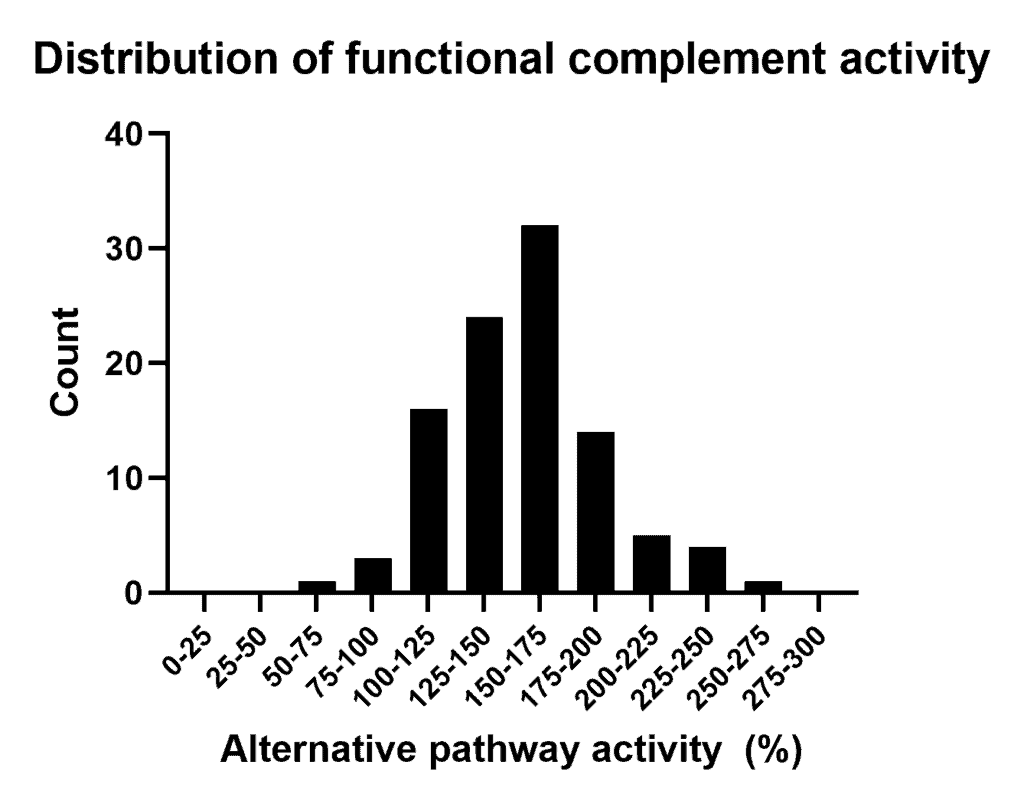
The assay has been designed in particular to test potential inhibitors or regulators on their activity. Most reliable results will be obtained when multiple concentrations of the inhibitor/regulator are tested against your own reference sample (see example figure).
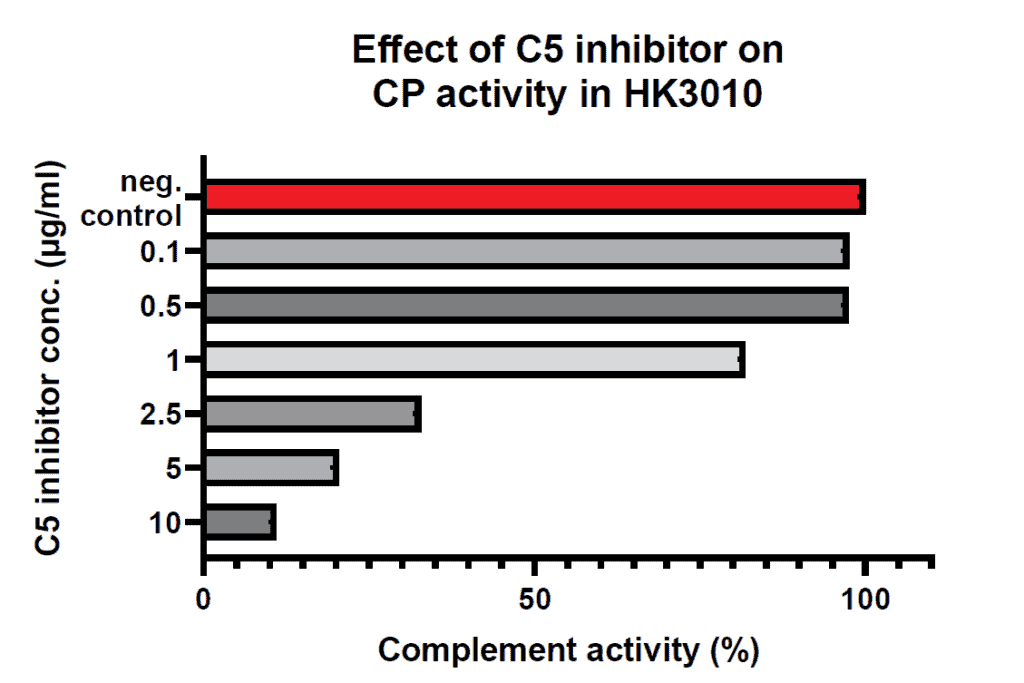
- I have performed the assay at room temperature. Can I use the results?
The complement cascade relies on enzymatic activity and complex formation, which is most optimal at 37C. At lower temperature most likely you get a lower complement activity. Most likely results cannot be used.
- I have performed the incubation step with reference standard, controls and samples at different incubation period. Can I use the results?
The complement pathway assays are optimal at incubation time indicated. Shortening the protocol is not advised, as this leads to a strong decrease in complement activity. Prolonging the incubation time leads to overactivation leading to too high OD values.
- Can I use other sample types than serum?
Whole blood samples might be useful, but have not been validated. Side effects could be expected. More insights on current guidelines for assessing complement activation can be found in the following paper: Brandwijk, R et al; Pitfalls in complement analysis: A systematic literature review of assessing complement activation. Front Immunol 2022; 13: 1007102.
Plasma samples cannot be used.
- Can I use historical serum samples?
Preferably use fresh serum samples. Historical samples can also be used, the quality of the result might depend on handling and storage conditions. More insights on current guidelines for assessing complement activation can be found in the following paper: Brandwijk, R et al; Pitfalls in complement analysis: A systematic literature review of assessing complement activation. Front Immunol 2022; 13: 1007102.
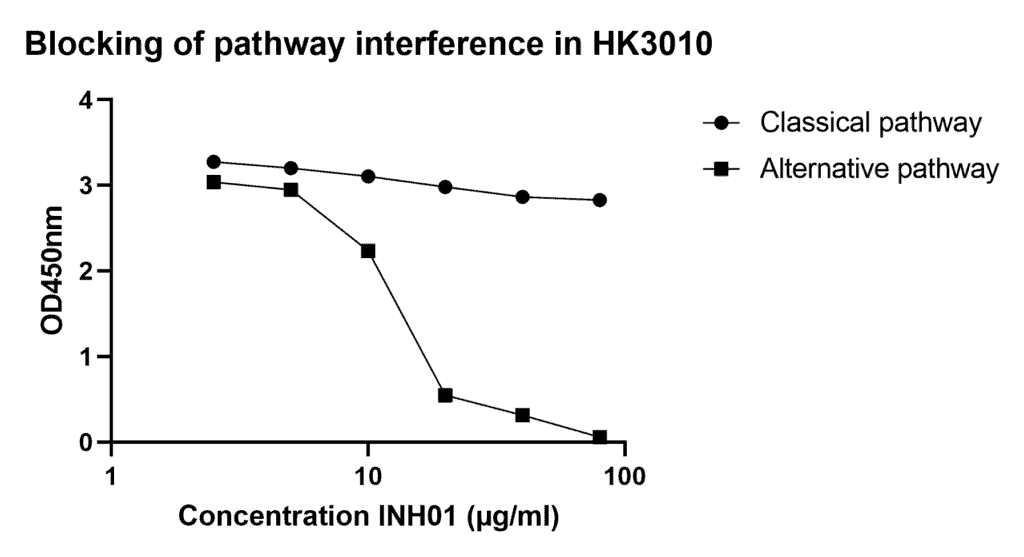
- Can I expect cross-interference of any of the other complement pathways?
By making use of pathway specific buffers and inhibitors we have blocked cross-interference of the other complement pathways. See figure below for an example of inhibition of the alternative pathway interference using INH01 in classical pathway assay (cat.# HK3010).
- Can I use the complement pathway assays for non-human primates (NHP)?
Both complement pathway assays (cat.# HK3010 and HK3012) have been validated on cynomolgus monkey serum samples. Measuring complement activity in cynomolgus monkey serum has to be optimized since the key-point dilution is at higher dilution compared to human serum samples. Please note that other NHP samples have not been tested, but are most likely useful because of expected high homology. If you are interested in using the assays for NHP and need custom adaption of protocol or assay format, custom services can be discussed. For that purpose, please contact us at support@hycultbiotech.com.
Antibody and protein FAQ
- What is the immunogen used?
The immunogen (either sequence or material used) is sometimes either not known or confidential. In those cases the information on the immunogen is not available. If the information is known and not classified, we include this information in the CoA-TDS. If there is no information in this document, you can always check with the technical support team to verify if there is any information that we can share based on your particular situation.
- Is the antibody available with a particular conjugate or label?
If they are available, you can always find them on the product page of the unlabeled version under the section “You may also be interested in the following product(s)”. We have antibodies conjugated with biotin, FITC and R-PE. If a product is not available with a particular conjugate or label, we can perform a conjugation based on your research needs. Contact our support team to learn more about the possibilities and pricing of these services. Note that we start offering custom products starting from 1 mg onwards.
- Are Hycult Biotech antibodies and proteins sterile?
All critical production processes such as purification and filling take place under aseptic conditions. Even though we cannot claim complete sterility we strive for the highest standards when it comes to the prevention of contamination and sterility.
- Are Hycult Biotech antibodies and proteins tested for endotoxins?
All antibodies that have a functional application are tested on endotoxins. If a product is tested on endotoxins, this exact concentration will be mentioned on the CoA-TDS when a product is purchased.
- What is the optimal working dilution?
The optimal working dilution depends on the nature of the product or the detection system applied. It is recommended that users test the reagent and determine their own optimal dilutions. The typical starting working dilution is 1:50 for most of our antibodies.
Shipping and storage FAQ
- The product is shipped under ambient conditions, will this have an impact on the quality of the product?
If a product is shipped under ambient conditions this does not have any influence on the product. Once the products have been received we advise to store them under the recommended storage conditions. There are some exceptions as we do have some products (e.g. complement proteins) that are shipped using dry ice.
- How does Hycult Biotech ship its products?
Hycult Biotech mainly works with FedEx as most shipments are dispatched using FedEx Express. Distributors may decide to work with other freight forwarders depending on preference, local circumstances or geographic conditions. Products are shipped under Ex Works conditions.
- Does Hycult Biotech ship to sanctioned countries?
Hycult Biotech fully complies with international sanctions and trade regulations. If a country is sanctioned, we closely work with government authorities to ensure trade takes place in a legitimate way.
- Do Hycult Biotech products contain hazardous materials as classified in the CLP guidlines?
Hycult Biotech works according to the REACH guideline (directive 1907/2006/EC) that replaces previous legislation on the regulation and classification of chemicals. All products that are classified as hazardous and require labeling have the necessary danger symbols based on the CLP regulation (1272/2008). The harmonized tariff code (HS code) for Hycult Biotech products is 3822.00.
- Original supplier of innate immunity products since 1994.
- Our commitment to quality: Read more!
- Contact your local distributor for ordering.
- Contact our support team for more information.
Calculate your ELISA data easily
With the ELISA calculator you can easily calculate ELISA data. Assayfit Pro helps to perform curve fitting. The calculator generates advanced reports, fit graph, fit parameters and goodness of fit are shown.
