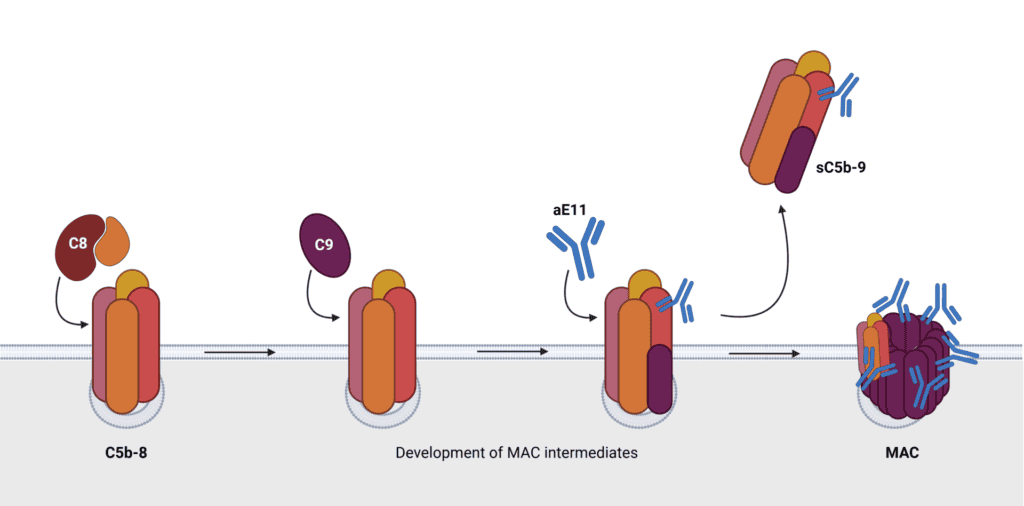
Basically, the Terminal Complement Complex (TCC) and Membrane Attack Complex (MAC) resemble the same protein complex. The complex can exist as membrane bound variant as well as a soluble variant, respectively in general referred to as MAC and soluble TCC (sTCC).
Introduction
The complement system is a distinguished part of the innate immune system. The complement system was initially described in the late 19th century by Jules Bordet, who observed that blood serum contains components that enhance antibody-mediated bacterial lysis. Subsequent research revealed that complement activation culminates in the formation of the MAC. This complex cascade consists of »50 proteins and can be triggered by three activation pathways (classical, lectin and alternative) and indirect by the extrinsic pathway of blood coagulation. Besides elimination of pathogens by direct lysis through MAC formation, the complement system contributes to numerous auto-immune and inflammatory diseases, like SLE, AMD and transplant rejection. Furthermore, it contributes in the activation of the adaptive immune response. Activation leads to the formation of the central C3 convertase, thereby propagating the proteolytic cascade to the formation of C5b from C5 by the C5 convertase. C5b is considered the start of the terminal pathway (TP) of complement, eventually leading to TCC. TCC is a barrel-shaped structure that inserts itself into the cell membrane, thus creating a pore.
Mechnanism and Structure
During the TP, while still attached to the convertase, the sequential addition of C6, C7 to C5b leads to the induction of conformational changes. This addition results into release from the convertase. After membrane association, this opens the ability to bind C8 and multiple copies of C9. This process is quite complicated and inefficient, in that way releasing a soluble nonlytic form of TCC. This so called C5b-9 complex or sTCC represents the failure rate of MAC assembly.
Looking at this process in more detail it turns out to be a quite sophisticated. After binding of C7 to C5b-6, the conformational change exposes a hydrophobic domain enabling association with the lipid membrane. This association is a highly inefficient process and can among others be interfered by regulatory proteins clusterin and vitronectin, which can mask this domain. It turns out the majority of the C5b-7 complexes fails to associate with the membrane. This offers the opportunity of binding of C8 and C9 in the fluid phase before membrane integration, leading to the formation of sTCC. When C5b-7 complexes do bind to the membrane, this can add a single copy of C8. This protein consists out of different subunits (α,b&g). The C5b-8 complex does not yet disrupt the membrane bilayer. The formation of the barrel through the bilayer acts in a specific order and rotation. C8 introduces a sequence of conformational changes. C8g on the interior of the barrel positions C8α in such way C9 can bind. The specific orientation of C8b to C8α enables incorporation of multi copies of C9 into the typical barrel shape of the pore. The number of C9 molecules in TCC can differ, from three copies up to eighteen.
Currently there is no cellular receptor known for sTCC and therefor there is no distinct function. Most consider sTCC as biologically inert. The amount of sTCC is considered to reflect the amount of MAC and as a measure of total complement activation or at least of the TP.
One way to interfere with complement activation is inhibition of MAC formation. CD59 is a glycosyl phosphatidylinositol (GPI-) anchored protein considered as a natural inhibitor of MAC arrangement. CD59 binds to C8 and C9, preventing incorporation into MAC. Other factors that play a regulatory role are decay-accelerating factor (DAF) and membrane cofactor protein (MCP). Understanding of these proteins offers therapeutic opportunities, but dysfunction or deficiencies can result in uncontrolled activation and can contribute to pathogenesis of disease.
Significance in disease and diagnosis
sTCC can be measured in plasma and other body fluids of healthy as well as diseased conditions. Multiple studies show changes in sTCC levels during infection, auto-immune disease and trauma. Although a clear function remains poorly understood, in patients suffering from infections sTCC has been proposed as a biomarker. It could reflect failure of incorporation or representation of the amount of lysed cells. In healthy individuals, there were no difference noticed between males and females, nor between Caucasians and African-Americans. However, above 50 years of age there seems to be an increase in concentration.
To quantify complement activation levels different diagnostic tools have been developed. Such recognized immunoassays make in most cases use of monoclonal antibodies detecting a neo-epitope present on an activation product and absent on their native counterpart. Antibody aE11 (HM2167) is such tool. It recognizes a neo-epitope present in C9 only after activation as represented in TCC or poly-C9. After structural studies, three critical residues were identified in C9 (R65, V68 and P72). These residues are available on top of the TCC molecule.
To ensure measurement of the endpoint of complement activation specifically, this antibody has been incorporated in several sTCC and pathway ELISAs (e.g. HK328, HK3010/11/12). To standardize the measurement of sTCC, the International Complement Standardization Committee has defined an activation standard for complement termed #ICS2. This standard is expressed as 1000 complement activating units/ml and can also be applied for sTCC. The measurement of sTCC has not been translated into common clinical use. However, with the emerging amount of new complement inhibitors making their way to the clinic in the coming years, it may be beneficial to use sTCC as a biomarker or companion diagnostics to monitor therapy and adapt dosing. This has been demonstrated for the use of Eculizumab which blocks the cleavage of C5 into C5a and C5b and thereby inhibiting the TP. Studies have showed that sTCC levels correlate well with Eculizumab dosing. This highlights its potential as a biomarker for monitoring complement inhibition and to be used for a personalized treatment plan.
Overall, advancements in structural analysis, diagnostic tools, and understanding of its regulation offer promising avenues for elucidating role of TCC in health and disease. The complex, particularly its soluble form sTCC, presents a fascinating yet complex entity in immune biology, holding promise for improved diagnostics and therapeutic interventions in various pathological conditions.