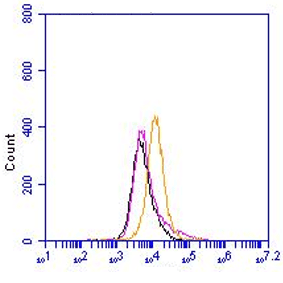
SAA-1, Human, mAb Reu86.1
€133.00 – €1,245.00
The serum amyloid A (SAA) family comprises a number of differentially expressed apolipoproteins, acute-phase SAA1 and SAA2, the former being the major component in plasma, and constitutive SAAs (C-SAAs). Although the liver is the primary site of synthesis of both SAA types extrahepatic production has been reported. The in vivo concentrations increase by as much as 1000-fold during inflammation. Several studies have stressed its importance in the diagnosis and monitoring of various diseases. Pathological SAA values are often detected in association with normal CRP concentrations; SAA rises earlier and more sharply than CRP.
Recently, a broader view of SAA expression and function has been emerging. Expression studies show production of SAA proteins in histologically normal, atherosclerotic, Alzheimer, inflammatory, and tumor tissues. SAA has been found to have binding sites for high density lipoproteins, calcium, laminin, and heparin/heparan-sulfate. Also adhesion motifs were identified and new functions, affecting cell adhesion, migration, proliferation and aggregation discovered. These findings emphasize the importance of SAA in various physiological and pathological processes, including inflammation, atherosclerosis, thrombosis, AA-amyloidosis, rheumatoid arthritis, and neoplasia. SAA has also a number of immunomodulatory roles, it can induce chemotaxis and adhesion molecule expression, has cytokine-like properties and can promote the upregulation of metalloproteinases. It enhances the binding of high-density lipoprotein to macrophages and thus helps in the delivery of lipids to sites of injury for use in tissue repair. It is thus thought to be an integral part of the disease processes. In addition, recent experiments suggest that SAA may play a “housekeeping” role in normal human tissues. Elevated levels of SAA over time predispose to secondary amyloidosis, extracellular accumulation of amyloid fibrils, derived from a circulating precursor, in various tissue and organs. The most common form of amyloidosis occurs secondary to chronic inflammatory disease, particularly rheumatoid arthritis. The antibody is raised against human SAA and Helix Pomatia Haemocyanine. It reacts specifically with SAA-1, the major isoform of SAA in plasma.