ATP7A, Mouse and Rat, pAb
€133.00 – €510.00
Rabbit polyclonal antibody CT77 reacts with mouse and rat ATP7A. Copper is essential for human health and copper imbalance is a key factor in the aetiology and pathology of several neurodegenerative diseases. Copper uptake into cells is thought to be mediated by the plasma membrane protein CTR1. Metallochaperones also bind copper and target it to specific destinations within the cell. ATOX1 (HAH1) transfers copper to the copper-ATPases. Copper-transporting ATPases (Cu-ATPases) ATP7A and ATP7B are evolutionarily conserved polytopic membrane proteins with essential roles in human physiology. The Cu-ATPases are expressed in most tissues, and their transport activity is crucial for central nervous system development, liver function, connective tissue formation, and many other physiological processes. These proteins have a dual role in cells, namely to provide sufficient amounts of essential intracellular copper and to mediate the excretion of excess of intracellular copper. ATP7A and ATP7B are members of a large family of P-type ATPases that are energy-utilizing membrane proteins functioning as cation pumps. They are called ‘P-type’ ATPases, as they form a phosphorylated intermediate during the transport of cations across a membrane. The domains involved in the catalytic cycle of the protein are the nucleotide-binding domain (N-domain), phosphorylation domain (P-domain), and activation domain (A-domain). ATP7A is anchored to a membrane through eight hydrophobic transmembrane domains, which form a channel for copper translocation through the membrane. At the N-terminus ATP7A has six metal-binding domains (MBD1–6) each with a consensus MTXCXXC motif. Copper binds to these domains in the reduced form, Cu(I). It is assumed that the two MBDs (MBD5 and MBD6) closest to the transmembrane domains are important for the functional activity of the protein, and at least one of these two sites is necessary for normal function of the protein. The first four metal-binding domains (MBD1–4) are thought to have a regulatory function. Interaction between ATP7A and the copper chaperone ATOX1 occurs through these domains. ATP7A is expressed in almost every organ except the liver where ATP7B is predominantly expressed. In concordance with this, copper is incorporated in ceruloplasmin by ATP7B in hepatocytes, while ATP7A is in charge in most other cell types in transporting copper to tissue-specific enzymes. The malfunctioning of copper homeostasis is demonstrated in Menkes disease in which the ATP7A gene is defective. Menkes disease results in copper accumulation in intestinal cells, placenta, mammary tissue and the kidneys and deficiency in the brain, liver and serum. This leads to disrupted neurological and connective tissue development, causing mental retardation and neurodegeneration and usually results in early childhood death. Disturbances in copper homeostasis are also associated with neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease, age-related macular degeneration and prion-related disease.
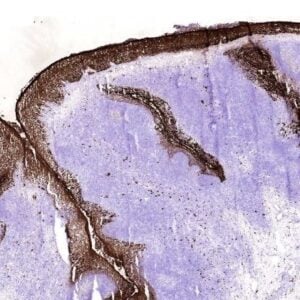
Polyclonal antiserum CT77, raised against the C-terminal end of ATP7A, recognizes the full length protein.
P: Fixed in Bouin's solution for 20h (4°C) and paraffin embedded. Endogenous peroxidase activity was blocked with 0.1% H2O2 in methanol (15 min).
W: A reduced sample treatment and SDS-Page was used.