New Antibody for Detection of the Membrane Attack Complex
The three distinct complement activation pathways converge at the formation of C3 convertase. This leads to the generation of C3b which triggers the assembly of the C5 convertase. This cleavage of C5 initiates the lytic, or terminal, pathway. Unlike the activation pathways that depend on enzymatic cleavage, the terminal pathway activation is driven by conformational changes upon binding. When C6 binds to C5b, it enables the subsequent binding of C7, which induces structural changes in the complex. The subsequent association of C8 allows multiple C9 molecules to bind to the C5b-8 complex, forming C5b-9, also known as MAC. The Terminal Complement Complex (TCC) and the Membrane Attack Complex (MAC) represent the same protein complex, existing as either membrane-bound MAC or soluble sTCC (sC5b-9).
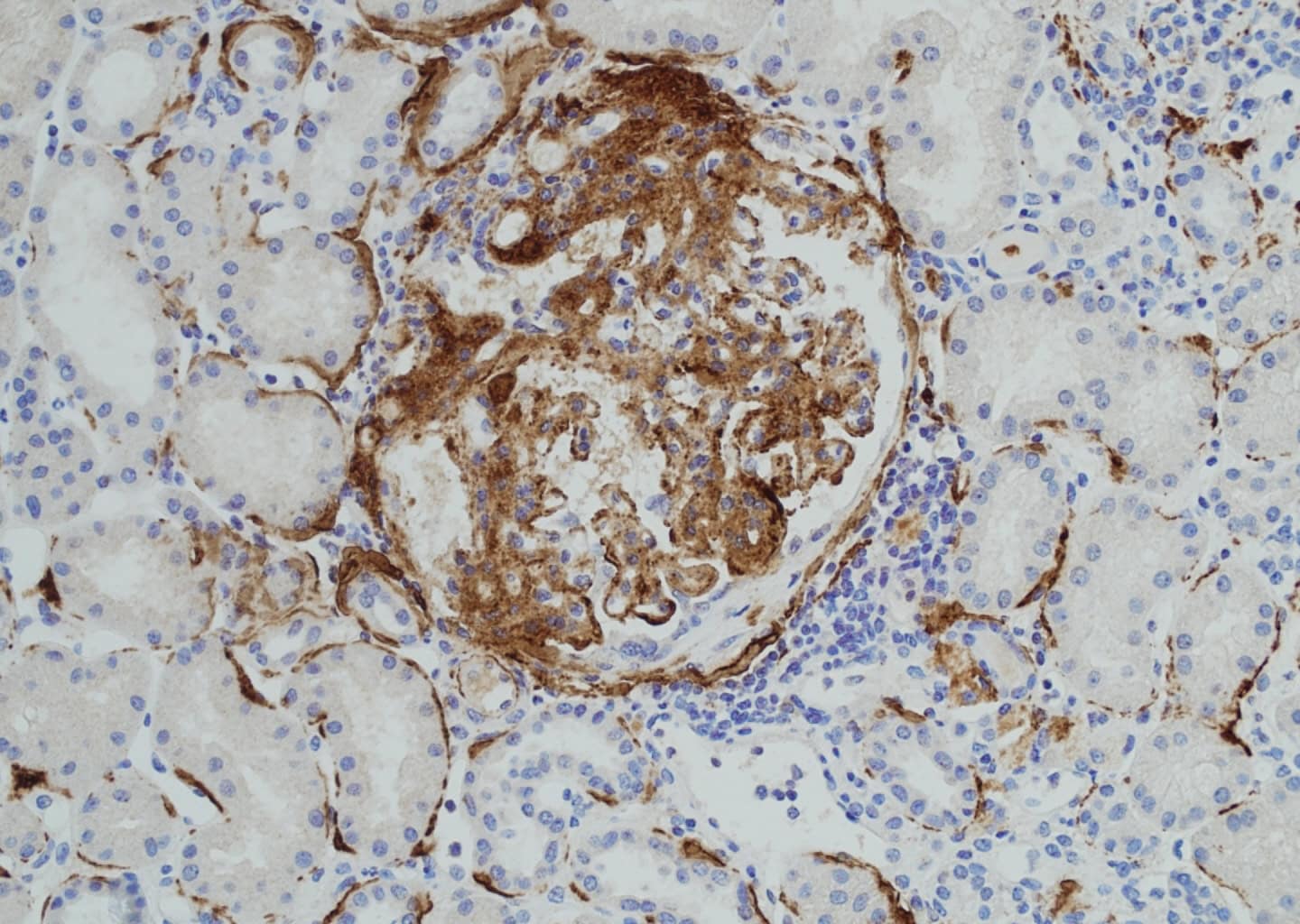
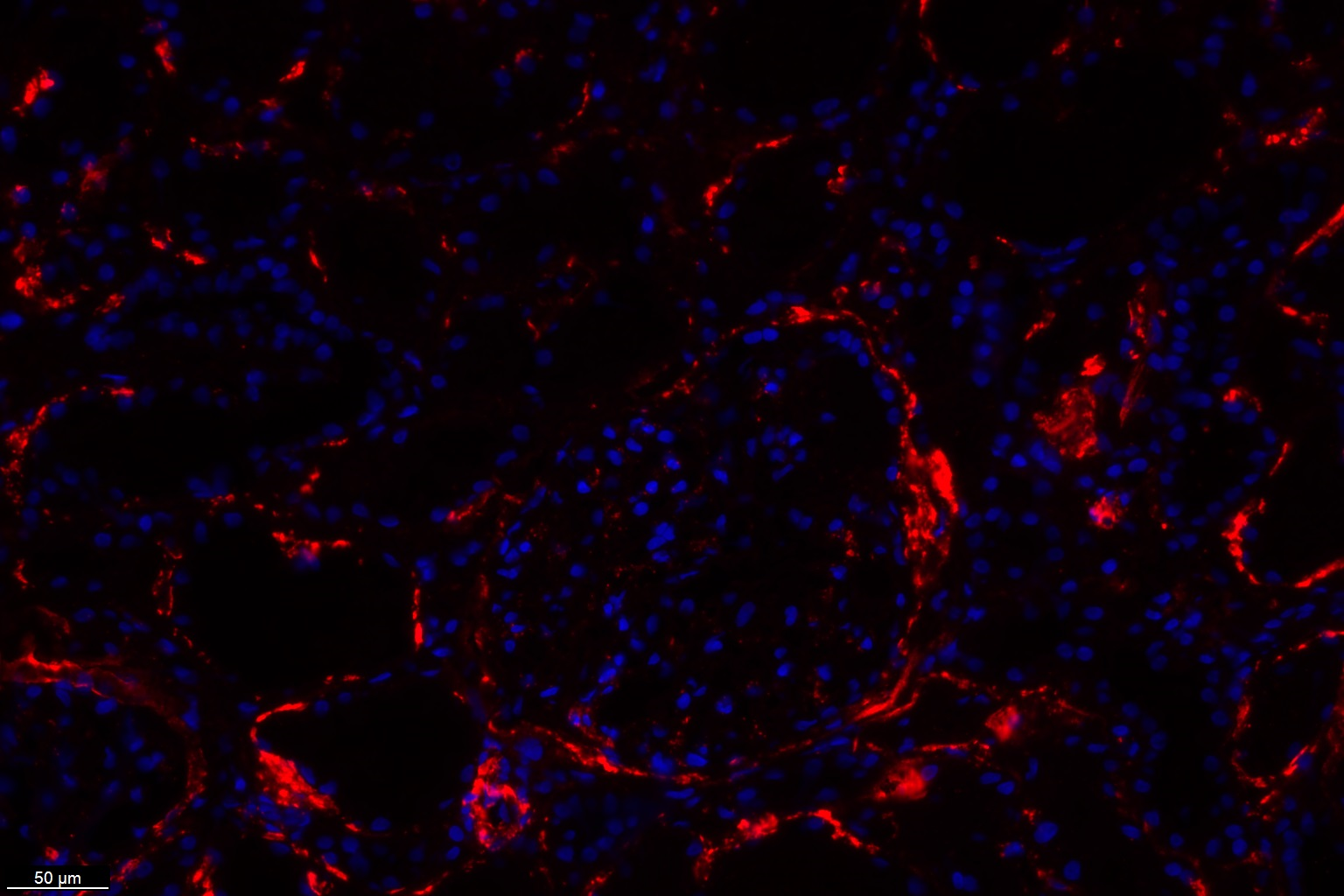
MAC formation results in cell lysis when the incorporated C9 molecules assemble into a pore-forming structure. This monoclonal B7 antibody (HM2443) specifically recognizes a C9 epitope within MAC. The B7 antibody is suitable for detecting C5b-9 (MAC) deposition in tissues, as shown in Figure 1 and 2, and is also capable of recognizing both C9 and soluble C5b-9 (sC5b-9/TCC) in fluid-phase samples, such as serum or plasma.