GEF-H1, Human, mAb B4/7
€133.00 – €565.00
The monoclonal antibody B4/7 recognizes human and canine guanine nucleotide exchange factor H1 (GEF-H1). GEFH1 is an ~110 kDa protein belonging to the Dbl family of proto-oncogenes. GEF-H1 can activate the small GTPase RhoA, but not Rac1 or Cdc42. Rho family GTPases are central regulators of epithelial tight junctions and the cytoskeleton. GEF-H1 can associate with different cytoskeletal structures, namely microtubules and the actin cytoskeleton. It has also been proposed to mediate crosstalk between the two types of filaments.
Localization of GEF-H1 differs between cell types. In MRC-5 fibroblast cells, GEF-H1 localizes to stress fibers. In epithelial cells, GEF-H1 is associated with apical tight junctions and involved in regulating paracellular permeability of small hydrophilic tracers. Furthermore, its subcellular localization changes in mitotic cells, where endogenous GEF-H1 is concentrated at mitotic spindles. GEF-H1 is capable of binding to the F-actin binding junctional adaptor cingulin. Binding to cingulin inhibits GEF-H1 and results in the downregulation of RhoA and inhibition of G1/S phase transition. In low confluent cultured cells, the localization of GEF-H1 is predominantly cytoplasmic. With increasing density of the cells, free GEF-H1 is sequestered at the tight junctions by cingulin.
GEF-H1 is part of the signaling pathway connecting epithelial polarity with the cell cycle, and as such involved in oncogenesis.
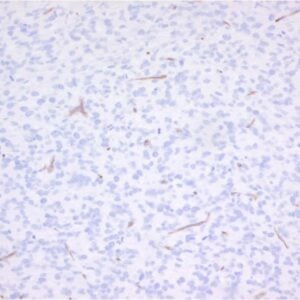
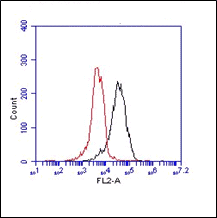
F: Cells were either fixed with methanol at -20 °C or with 3% PFA followed by permeabilization with Triton X-100.




Related products
-
View product €133.00 – €320.00
-
View product €133.00 – €456.00
-
View product €380.00
-
View product €133.00 – €320.00
-
View product €510.00
-
View product €133.00 – €414.00
-
View product €133.00 – €276.00
-
View product €359.00