CRAMP, Mouse, mAb LF7-BB3
€133.00 – €456.00
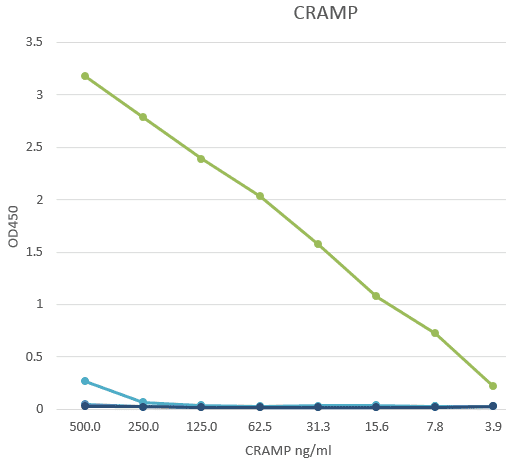
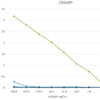
The monoclonal antibody LF7-BB3 recognizes the mouse protein Cathelin-Related AntiMicrobial Peptide (Cramp). Cathelicidins are a familiy of antimicrobial proteins predominantly found in the peroxidase-negative granules of neutrophils. the cathelicidins are synthesized as preproteins. Within the neutrophils, they are stored in granules as inactive proforms after removal of the signal peptide. The biologic active domains of the cathelicidins reside in the C-terminus. The C-terminal antimicrobial peptides are activated when cleaved from the proforms of the cathelicidins by serine proteases from azurophilic granules. Cramp (Cathelin-Related Anti-Microbial Peptide) is the mouse analogue of human LL-37 peptide, which is the antibacterial C-terminus of hCAP-18 (human cathelicidin). CRAMP forms an amphipathic α-helix similar to other antimicrobial peptides. Cramp is a potent antibiotic against Gram-negative bacteria by inhibiting growth of a variety of bacterial strains and by permeabilizing the inner membrane of E.coli directly. Abundant expression of Cramp is found in myeloid precursors and neutrophils. Cramp represents the first antibiotic peptide found in cells of myeloid lineage in the mouse. Inflammatory cells in the mouse can thus use a non-oxidative mechanism for microbial killing. The monoclonal antibody LF7-BB3 is suitable as a capture and/or detection in an immunoassay (ELISA).
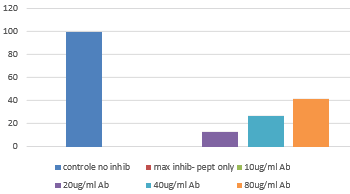
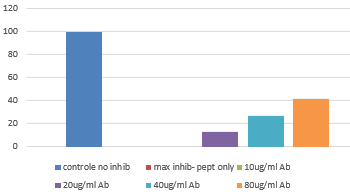
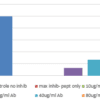
- FS: The bacterial activity of cramp peptide can be blocked with HM1127 in a dose dependent way.
- HM1127 cannot be used for Western blot.