EPCR, Human, mAb RCR-379
€125.00 – €584.00
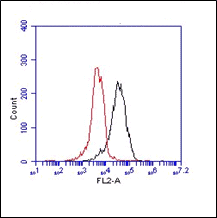
The monoclonal antibody RCR-252 recognizes human endothelial protein C receptor (EPCR), a highly glycosylated type I transmembrane protein of 221-amino-acids. These amino acids comprise an extracellular domain, a 25-aa transmembrane domain, and a short (3 aa) intracytoplasmic sequence coding for an ~46 kDa protein. Deglycosylation will reduce the protein mass to 25 kDa. EPCR is expressed strongly on the endothelial cells of arteries and veins in heart and lung, less intensely in capillaries in the lung and skin, and not at all in the endothelium of small vessels of the liver and kidney.
EPCR is the receptor for protein C, a key player in the anticoagulation pathway. The protein C anticoagulant pathway serves as a major system for controling thrombosis, limiting inflammatory responses, and potentially decreasing endothelial cell apoptosis in response to inflammatory cytokines and ischemia. The essential components of the pathway include thrombin, thrombomodulin, the endothelial cell protein C receptor (EPCR), protein C and protein S. The pathway is initiated when thrombin binds to thrombomodulin on the surface of endothelium. EPCR augments protein C activation by binding protein C and presenting it to the thrombin-thrombomodulin activation complex. Activated protein C (aPC) retains its ability to bind EPCR, and this complex appears to be involved in some of the cellular signaling mechanisms that down-regulate inflammatory cytokine formation (TNF, IL-6). EPCR is shed from the vasculature by inflammatory mediators and thrombin. EPCR binds to activated neutrophils in a process that involves proteinase 3 and Mac-1. Furthermore, EPCR can undergo translocation from the plasma membrane to the nucleus.
EPCR can be cleaved to release a soluble form (sEPCR) in the circulation. This sEPCR is detected as a single species of 43 kDa, resulting from shedding of membrane EPCR by the action of a metalloprotease,- which is stimulated by thrombin and by some inflammatory mediators. Soluble EPCR binds PC and aPC with similar affinity, but its binding to aPC inhibits the anticoagulant activity of aPC by blocking its binding to phospholipids and by abrogating its ability to inactivate factor Va. sEPCR- can be detected in plasma. In normal persons, sEPCR is present in levels of 83.6 +/- 17.2 ng/ml. Elevated levels of sEPCR are positively correlated to a higher risk for thrombosis. Furthermore, a haplotype (A3 allele) has been linked to elevated levels of sEPCR (264 +/-174 ng/ml).
Calculate your ELISA data easily
With the ELISA calculator you can easily calculate ELISA data. Assayfit Pro helps to perform curve fitting. The calculator generates advanced reports, fit graph, fit parameters and goodness of fit are shown.
We are glad to support you!
Take advantage of our dedicated support team for any technical assistance you need while using our products or considering them for your research needs.