FHL2, Human, mAb F4B2-B11
€133.00 – €620.00
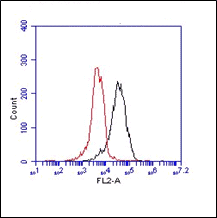
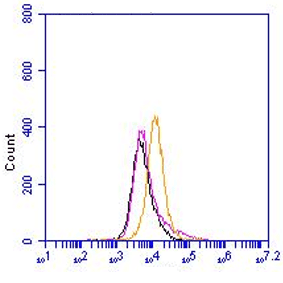
The monoclonal antibody F4B2-B11 reacts specifically with the LIM-only protein FHL2. Five proteins share the same structural organization and a high degree of sequence homology in this group: four and a half LIM domain protein (FHL) 1, FHL2, FHL3, FHL4, and activator of cAMP-responsive element (CRE) modulator (CREM) in testis (ACT). LIM domains are constituted by a conserved cysteine- and histidine-rich structure shaped in two repeated zinc fingers first identified in the proteins encoded by the Lin-11, Isl-1, and Mec-3 genes. The LIM domain has been shown to function as a protein-protein interaction domain, and has often been described in association with other functional protein motifs, such as homeobox and kinase domains. FHL2 seems to be a promiscuous coactivator because it modulates the activity of the androgen receptor, CRE-binding protein (CREB), and WT1, although some degree of specificity is present because it is unable to stimulate CREM- and Sp1-dependent transcription. FHL2 expression was originally described to be restricted to the heart but it is inducible in other cell types. FHL2 shows specific interaction with beta-catenin, which requires the intact structure of all four LIM domains of FHL2 and the N-terminus plus the first armadillo repeat region of beta-catenin. FHL2 is a muscle-specific repressor of LEF/TCF target genes and promotes myogenic differentiation by interacting with beta-catenin. Monoclonal antibody F4B2-B11 recognizes the N-terminal Zn-Finger motif, it does not crossreact in Western Blotting with the FHL1 and FHL3 proteins. The F4B2-B11 antibody is cross reactive with mouse and rat FHL2.