Complement Factor H: a key regulator of the complement system
Factor H (FH) is one of the most crucial complement regulators for the protection of human cells and surfaces from complement, and therefore of great interest to clinical research. FH regulates the alternative pathway (AP) by inhibiting the formation of the C3-convertase C3bBb in multiple ways. It belongs to a protein family consisting of 7 closely related proteins: FH, FH-like protein 1 (FHL-1), and five FH related proteins (FHR-1 to 5). In this overview, we provide information about FH and the FHRs, regarding their structure, function, and role in various diseases.
We are glad to support you!
Take advantage of our dedicated support team for any technical assistance you need while using our products or considering them for your research needs.
- Original supplier of innate immunity products since 1994.
- Our commitment to quality: Read more!
- Contact your local distributor for ordering.
- Contact our support team for more information.
What is complement Factor H (FH)?
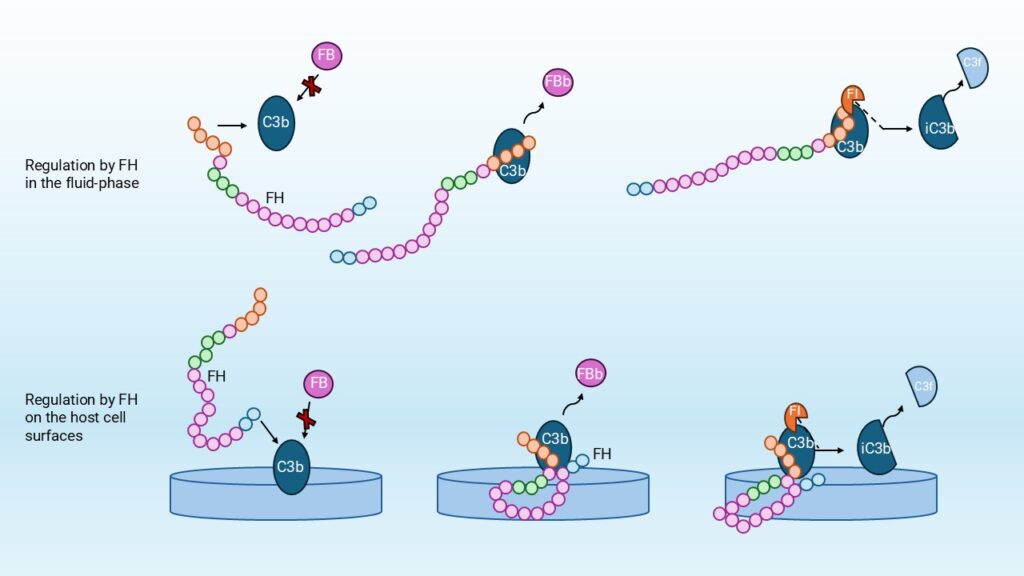
FH is the main regulator of the AP, acting both in the fluid phase and on cell surfaces. In the fluid phase, FH prevents excessive amplification of the complement system by specifically binding C3b, thereby preventing the formation of the C3bBb convertase complex, (Figure 1). Additionally, when FH is bound to the cell surface, it provides local protection by binding to host-specific surface markers such as sialic acids and glycosaminoglycans, which enables discrimination between self and non-self surfaces. This binding inhibits complement activation directly at the site of C3b deposition [1]. Further roles will be discussed in the upcoming paragraphs.
The FH protein family consists of seven proteins: FH, FHL-1, and five FHRs (FHR-1 to FHR-5). These proteins are primarily produced in the liver, but extra-hepatic production has also been reported [2]. FH is abundantly present in blood, with physiological concentrations ranging from 135 to 250 mg/L [3]. Next to their presence in plasma, these proteins are also detected in retinal pigment epithelial cells and glomerular mesangial cells. [2]
Structure of Factor H and FH-related proteins
FH is a plasma glycoprotein of 155 kDa and composed of 20 complement control protein (CCP) domains. CCPs are also referred to as short consensus repeats (SCRs), which are arranged in a ‘beads-on-a-string’ configuration [4][5]. Each CCP consists of approximately 60-65 amino acids stabilized by two disulfide bonds.
The FH protein family includes FH itself, its splice variant Factor H-like protein 1 (FHL-1), and five Factor H-related proteins (FHRs). Factor H like-1 (FHL-1) is an alternative splice variant of FH. FHL-1 consists of CPP domains 1-7, identical to those in FH, but alternative splicing gives it a unique four-amino acid C-terminal tail. FHL-1 displays different surface specificities in comparison to FH, mediated by its short consensus repeats 6 and 7 (SCR6-7) domains and the unique 4 amino acids at the C-terminus. [6]
The FHRs share significant sequence homology with FH but lack domains homologous to CCPs 1-4, which are required for complement regulation. All FHR proteins are composed entirely of CCP domains, and several of these domains are nearly identical at the amino acid level. Despite differences in overall length (ranging from four to nine CCP domains), many FHRs share highly similar regions, see Figure 2. For instance, FHR-1B contains a CCP domain that is 100% identical to CCP18 of FH, suggesting direct competition for similar ligands. Likewise, FHR-1, FHR-2, and FHR-5 share high sequence identity in their N-terminal domains, which enables dimerization and further increases their structural similarity to FH.
FHRs show a striking yet variable sequence similarity with FH, the separate domains ranging from approximately 32% to complete identically (100%) at the amino acid level, (Figure 2). This homology is most pronounced in domains involved in ligand recognition, particularly CCPs 6-8 and 19-20 of FH. FHR proteins consist exclusively of CCP domains, structurally resembling those in FH, but they lack the regulatory domains necessary for complement inhibition. Variations in glycosylation among the FHRs further influence molecular weight and possibly binding behavior, but overall, their domain architecture reveals that the FHR family consists of closely related proteins with overlapping structural elements derived from FH [6].
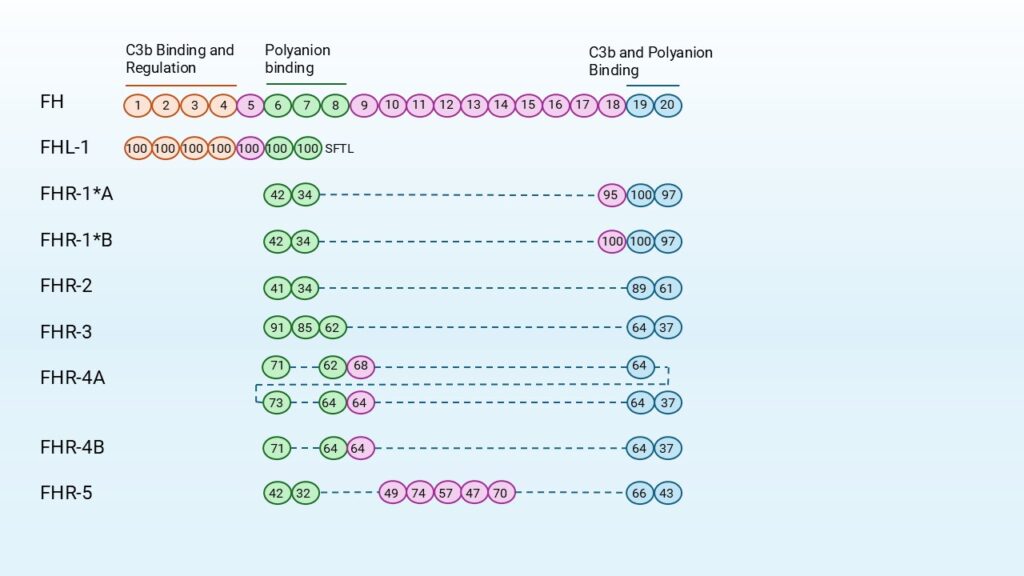
Based on domain composition and sequence similarity, the FHR proteins can be divided into two subgroups:
- The first group includes: FHR-1, FHR-2, and FHR-5. These share high sequence identity (42–100%) in CCP domains 1 and 2. The N-terminal domains contain a dimerization motif, enabling these proteins to form both homo- and heterodimers.
- The second FHR subgroup consists of FHR-3 and FHR-4. These lack dimerization motifs and do not form oligomers. They do, however, share high sequence identity in their N-terminal domains. No evidence exists for protein-protein interactions within this group.
Among the FHRs, FHR-5 is structurally unique because it contains domains highly homologous to CCPs 10–14 of FH, a region involved in ligand interactions. This may give FHR-5 specific binding properties that influence complement activation and regulation. [4]
Role of Factor H and FH-related proteins
FH regulates the AP by inhibiting the formation of the C3-convertase C3bBb in four ways. First, FH prevents the formation of new C3bB complexes by competing with factor B (FB) for binding to C3b. Secondly, FH acts as a decay-accelerating factor (DAF), promoting the decay of pre-formed C3bBb convertases. Lastly, FH functions as a cofactor for the FI-mediated cleavage of C3b into inactivated C3b (iC3b), irreversibly inactivating C3b and preventing further AP amplification. In addition, FH blocks FB from forming C3(H2O)Bb and C3bBb convertases.
The regulatory function is linked to the structure of FH itself. The CCP domains are each mediating distinct functional activities. The N-terminal CCPs 1–4 control complement activity; they are responsible for the competition with FB, the decay-accelerating activity, and cofactor activity for factor I (FI)-mediated cleavage of C3b.
Other regions of FH, particularly CCPs 6–8 and the C-terminal 19–20 enable FH to bind ligands. These include C3b, glycosaminoglycans (GAGs) and C-reactive protein (CRP). These interactions, particularly with GAGs and C3b, are crucial for the ability of FH to localize to host tissues and inhibit complement activation at those sites, thereby protecting the host cells from excessive inflammation and tissue damage.
FHL-1 possesses complementary regulatory functions similar to FH. However, since it lacks the domains required for host surface recognition, it primarily acts as a regulator in the fluid phase. [4]
To complete the family, there are five FHRs: FHR-1, FHR-2, FHR-3, FHR-4 and FHR-5. In contrast to FH, it is suggested that they can enhance complement activation by competing with FH for binding factors such as C3b and glycosaminoglycans [8]. Despite their sequence homology, FHRs lack the regulatory domains required for complement inhibition. Their ability to modulate complement activation by displacing FH highlights their role in the modulation of immune responses. However, their precise biological role remains poorly understood, underlining the need for continued research into the contribution of the FH protein family to immune system regulation [4] [6].
The Role of CFH and CFHR Genetic Variants in Human Diseases
Genetic variants of CFH and CFHR have been widely studied because some can significantly influence susceptibility to multiple diseases [7]. These variants may involve single nucleotide polymorphisms, copy number variations, or gene rearrangements, which can affect the function or expression of FH and FHR proteins [7]. As a result, dysregulation of complement activity can occur, contributing to the development of autoimmune, infectious, renal, ocular, neurodegenerative, and even malignant disorders.
In the context of cancer, many tumors have been shown to overexpress FH as a mechanism to evade immune surveillance. By inhibiting complement activation, FH protects tumor cells from complement-mediated lysis. High FH expression has been correlated with poor prognosis in several cancer types, including lung, ovarian, and colorectal cancer [2][9].
Similarly, vascular diseases such as cardiovascular disease (CVD) have been associated with dysregulated FH expression or activity. This imbalance may influence complement-mediated inflammation within blood vessels, thereby contributing to vascular injury and disease progression [2].
Mutations in CFH often impair FH’s ability to bind to host cell surfaces, thereby reducing protection against complement-mediated damage. A well-known example of a complement-mediated autoimmune disease is atypical hemolytic uremic syndrome (aHUS). aHUS is a rare, life-threatening renal disease characterized by uncontrolled complement activation leading to hemolytic anemia, thrombocytopenia, and kidney injury.
Similarly, deletions in CFH and CFHR3 have been associated with increased invasive meningococcal disease and other infections caused by Neisseria species [7]. In renal pathology, C3 glomerulopathy (C3G) has also been associated with CFHR3–CFHR1 deletions, which lead to an imbalance between FH and FHR proteins. This imbalance diminishes complement regulation and promotes the deposition of complement fragments in the glomeruli, driving inflammation and tissue injury.
In the eye, age-related macular degeneration (AMD) represents another condition associated with CFH variants, particularly the Y402H polymorphism in FH, which alters its interaction with host ligands in retinal tissue. Beyond these, emerging evidence links CFH polymorphisms to neurodegenerative diseases such as Alzheimer’s disease (AD), where altered complement regulation contributes to chronic neuroinflammation and accelerates neuronal damage [2][4][7].
Together, these diverse disease associations highlight the role of FH in maintaining complement homeostasis and underline the importance of measuring FH and its related proteins. This to better understand their involvement in disease mechanisms. Nevertheless, there are still challenges in FH and FHRs since there is, for example, a lot of overlap in structure. The following section explores these challenges in more detail and outlines recommendations for advancing research in this field.
Challenges in Factor H and its Related Proteins measurements
Research on FH and FHRs can be challenging, because of their strong structural resemblance, which complicates differentiation between family members. Hycult’s dedicated assays overcome this challenge, they have been carefully validated for specificity and proven ability to distinguish between FH and its related proteins.
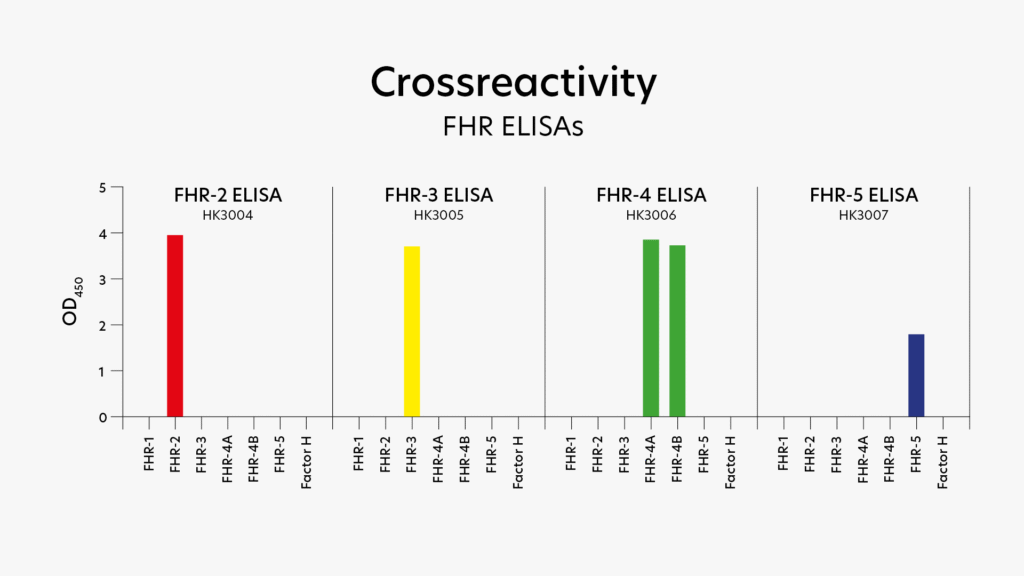
We developed validated ELISAs for FHR-2, FHR-3, FHR-4 and FHR-5 that do not cross-react with other members of the FH family, (Figure 3)[8]. In addition, Hycult offers ELISAs for Factor H and the Factor H 402H/Y variant.
Contact us for more information
At Hycult Biotech, we recognize the growing demand for advanced complement research tools that facilitate accurate analysis and targeted intervention in immune-related diseases. Our portfolio includes high-quality complement pathway inhibitors (how to inactivate complement), complement assay kits, and monoclonal antibodies designed to support drug discovery and translational research. On our website, you can search for products that match your research needs. Additionally, we are happy to provide expert advice. Feel free to contact us via the contact form.
Frequently asked questions
FH(Rs) can be assessed in different ways, depending on the purpose of the analysis: quantification and/or functional activity. When quantification is required, an ELISA can be used to measure the concentration of FH(Rs) in plasma. At Hycult Biotech, ELISAs are available for FH, Factor H 402H/Y variant, FHR-2, FHR-3, FHR-4, and FHR-5. The development of an assay for FHR-1 is currently under development and will be available soon. Cross-reactivity for FHR-2, FHR-3, FHR-4 and FHR-5 is extensively tested (Figure 3). Producing specific reagents is challenging due to the high structural similarity between the Factor H proteins, as illustrated in Figure 2. However, Hycult’s FHR assays are specific for their corresponding target protein.
- Competition with factor B: FH interferes with factor B (FB) for binding to C3b, thereby inhibiting the formation of new convertase complexes (C3bB).
- Decay-accelerating factor (DAF): FH enhances the dissociation of the AP C3 convertase (C3bBb), thereby reducing its half-life and reducing ongoing complement activation.
- Cofactor activity for factor I (FI): Possibly its most critical function, FH functions as a cofactor for FI-mediated cleavage of C3b into iC3b, an inactive form that cannot extend further complement activation.
- Inhibition of convertase formation: FH additionally blocks FB from forming C3(H2O)Bb and C3bBb convertases, further preventing excessive complement activation. [1] [4]
FH and FHRs proteins are central regulators of the alternative pathway. When the regulation is impaired, which might be caused by genetic mutations, autoantibodies or imbalances, dysregulation in complement activation can occur. Such dysregulation is linked to a wide spectrum of human diseases, below several examples:
- Kidney diseases: Atypical hemolytic uremic syndrome (aHUS) and C3 glomerulopathy (C3G) are examples where FH mutations or anti-FH autoantibodies cause the disease by dysregulation in complement activation on renal endothelium [1] [2].
- Autoimmune diseases: In systemic lupus erythematosus (SLE) and ANCA-associated vasculitis FH dysregulation is implicated. In these diseases, overactivation exacerbates autoimmunity and inflammation [1] [2].
- Eye diseases: Genetic variants in FH genes and imbalances between FH and FHRs disrupt complement control in the eye, leading to ongoing inflammation and damage [2] [7].
- Cancer: Many tumors overexpress FH to protect themselves from immune attack. High FH expression correlates with worse prognosis in cancers like lung, ovarian, and colorectal cancer [2] [9].
- Vascular diseases: Dysregulated FH has been associated with cardiovascular disease (CVD), possibly by altering complement-mediated inflammation in blood vessels [2].
- Infectious diseases: Certain pathogens, like Neisseria meningitidis and Staphylococcus aureus, hijack FH to evade complement-mediated destruction, and individuals with low FH activity are more susceptible to severe infections [6].
Cobra venom factor (CVF) is a protein isolated from cobra species Naja naja and Naja haje. CVF has a potent effect on the complement system, in particular the alternative pathway. It mimics the structure and function of human C3b. It binds factor B, which is then cleaved by factor D. Together this forms a CVF-Bb complex that continuously cleaves C3 and C5. Unlike natural C3b-based convertases, which are regulated by factors H and I, this CVF-Bb complex is resistant to such regulation. CVF is used by researchers to study the role of complement in various disease models. It allows temporary complement depletion in vivo. This aids in investigating complement-related pathways.
[1] Saskia Nugteren, Haiyu Wang, Cees van Kooten, Kyra A. Gelderman, Leendert A. Trouw, Autoantibodies and therapeutic antibodies against complement Factor H, Immunology Letters, Volume 274, 2025, 107002, ISSN 0165-2478, https://doi.org/10.1016/j.imlet.2025.107002.
[2] Pratiti Banerjee, Bert R.J. Veuskens, Elena Goicoechea de Jorge, Mihály Józsi, Antje J. Baeumner, Mark-Steven Steiner, Richard B. Pouw, Erik J.M. Toonen, Diana Pauly, Felix Poppelaars, Evaluating the clinical utility of measuring levels of Factor H and the related proteins, Molecular Immunology, Volume 151, 2022, Pages 166-182, ISSN 0161-5890, https://doi.org/10.1016/j.molimm.2022.08.010.
[3] The complement Factsbook, second edition, Scott Barnum, Theresa Schein, 2018, chapter 30, Factor H and Factor H-like Protein 1.
[4] Poppelaars F, Goicoechea de Jorge E, Jongerius I, Baeumner AJ, Steiner M-S, Jo´ zsi M, Toonen EJM, Pauly D and the SciFiMed consortium (2021) A Family Affair: Addressing the Challenges of Factor H and the Related Proteins. Front. Immunol. 12:660194. doi: 10.3389/fimmu.2021.660194
[5] Marcell Cserhalmi, Alexandra Papp, Bianca Brandus, Barbara Uzonyi, Mihály Józsi, Regulation of regulators: Role of the complement factor H-related proteins, Seminars in Immunology, Volume 45, 2019, 101341, ISSN 1044-5323, https://doi.org/10.1016/j.smim.2019.101341.
[6] Richard B. Pouw, Setting the scale: The balance between complement Factor H and its related proteins in health and disease, 2018, Department of Immunopathology of Sanquin Research (Amsterdam, The Netherlands), ISBN: 978-94-9301-485-5
[7] Lucientes-Continente L, Márquez-Tirado B, Goicoechea de Jorge E. The Factor H protein family: The switchers of the complement alternative pathway. Immunol Rev. 2023 Jan;313(1):25-45. doi: 10.1111/imr.13166. Epub 2022 Nov 16. PMID: 36382387; PMCID: PMC10099856.
[8] van Rossum M, Veuskens BRJ, Brouwer MC, van Mierlo G, Lucientes-Continente L, Goicoechea de Jorge E, Uzonyi B, Matola AT, Józsi M, Müller G, Meter-Arkema AM, Poppelaars F, Pauly D, Pouw RB, Toonen EJM. Development and Characterization of Novel ELISAs for the Specific Quantification of the Factor H-Related Proteins 2, 3, 4, and 5. J Innate Immun. 2025;17(1):226-243. doi: 10.1159/000545139. Epub 2025 Mar 26. PMID: 40139166; PMCID: PMC12048131.
[9] Saxena R, Gottlin EB, Campa MJ, Bushey RT, Guo J, Patz EF Jr. and He Y-W (2024), Complement Factor H: a novel innate immune checkpoint in cancer immunotherapy. Front. Cell Dev. Biol. 12:1302490. doi: 10.3389/fcell.2024.1302490